Clinical team's uneven struggle with hospital reality
The world of clinical trials
Elevated data requirements, adherence to protocols, precise and comprehensive data.
- Inclusion and Exclusion Criteria
- Informed patient consent
- Case Report Form (eCRF)
- Research protocol
- Adverse events
- Study phase Randomization
=
Hospital reality
Focus on patient treatment and daily clinical tasks.
- Work overload
- Bureaucracy
- Documentation requirements
- Little time for the patient
- Adverse events
- Hospital systems not adjusted to trials
Problems arising from the mismatch of these two worlds
- Lack of unambiguous mapping between the source (usually a medical note) and eCRF
- Partial lack of the data required by, usually very extensive, eCRF
- Delays, excess amount of work = unnecessary costs
- Time-consuming process of entering data into eCRF
- Excess and unnecessary number of data query
- Risk of deviation from the protocol

Assisting doctors in managing the patient in accordance with study requirements
The world of clinical trials
Elevated data requirements, adherence to protocols, precise and comprehensive data.
- Inclusion and Exclusion Criteria
- Informed patient consent
- Case Report Form (eCRF)
- Research protocol
- Adverse events
- Study phase Randomization
*Automatic export of the study structure

Platform
*The degree of automation depends on the type of electronic data collection system (EDC) you have in place
Easy search in the hospital system for the data needed to complete the eCrf
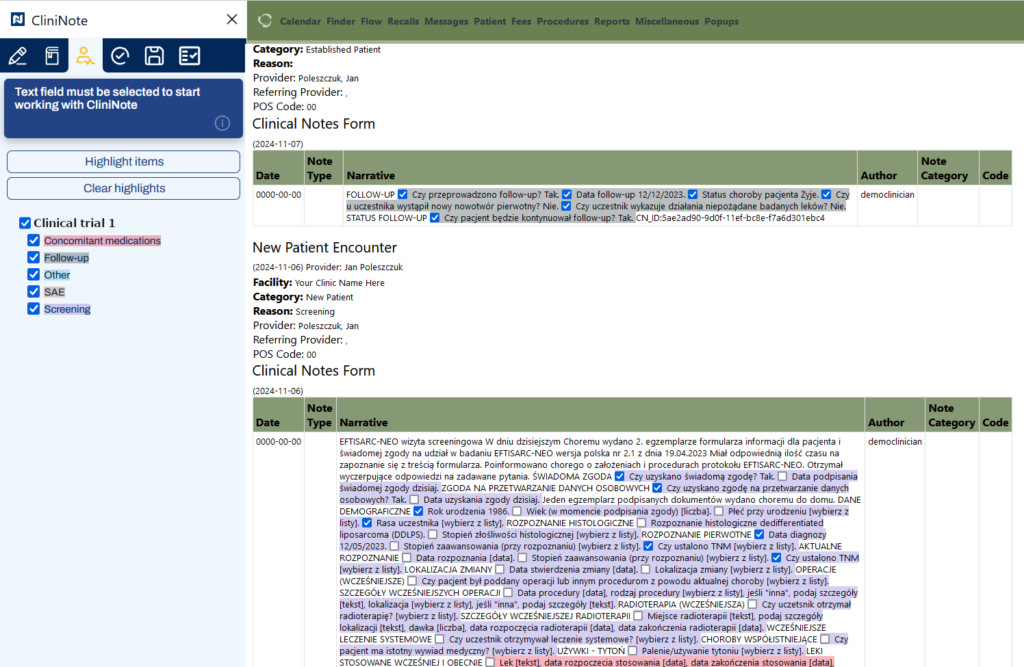
Notes elements directly related to eCRF clearly highlighted for data entry purposes*
*Requires installation of an additional component in the hospital
**Data input into eCRF can be automated depending on the hospital infrastructure
Process improvement with Clininote - added value
- Protocol structure mapping in notes
- All data required by eCRF collected during the visit
- Direct mapping between the source (medical note) and eCRF
- Significant time reduction of entering data into eCRF
Benefits
- Data query reduction
- Reduction of delays and work overload
- Reduction of clinical trials costs
How does CliniNote work with eCRF?
eCRF/register
form 1, 2, …, N
1.Exporting study metadata to CliniNote

2.CliniNote generates accurate visit templates
4.Study results are stored in the database (ready to by uploaded to eCRF)

3.Researchers use CliniNote Assistant during visits
3.Researchers use CliniNote Assistant during visits

4.Study results are stored in the database (ready to by uploaded to eCRF)

